Concomitant ALK rearrangements and TP53 mutations were associated with worse alectinib therapy outcomes in patients with non-small cell lung cancer (NSCLC) vs ALK rearrangements only, according to the results of a study published in Clinical Cancer Research.1 Data from in vitro and animal models suggest that proteasome inhibitors such as ixazomib may increase the efficacy of alectinib in the presence of coexisting ALK and TP53 alterations.
Alectinib is an ALK inhibitor that is now a standard first-line treatment for patients with advanced, ALK-rearranged NSCLC. Previous reports have suggested that the presence of both ALK and TP53 aberrations may reduce the efficacy of alectinib therapy in NSCLC. The aim of this study was to determine the effect of concomitant genetic alterations on resistance to ALK inhibition and how it might be overcome.
The study authors evaluated data from 7011 patients with NSCLC, with a focus on the 90 patients with ALK-rearranged NSCLC who received treatment with alectinib through LC-SCRUM-Japan, a nationwide lung cancer genome project. Tumor DNA and RNA were assessed by next-generation sequencing.
In in vitro studies, the investigators used EML4–ALK fusion-positive NSCLC cell lines to evaluate the role of p53 function and the activity of the proteasome inhibitor ixazomib. Mouse models with subcutaneous tumors generated from ALK-rearranged NSCLC with nonfunctional p53 were used to assess the efficacy of ixazomib plus alectinib.
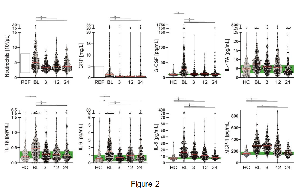
Of the 6444 evaluable patients, 2.5% harbored ALK fusions. Concomitant TP53 mutations were present in 25.0% of patients.
Among the 90 patients with ALK-rearranged disease who received alectinib, the presence of a TP53 mutation was found to result in a significant decrease in progression-free survival (PFS), with a median PFS of 11.7 months (95% CI, 6.3-not reached [NR]) in the concomitant alteration subset compared with a median PFS of NR in the ALK alteration alone group (95% CI, 23.6-NR; HR, 0.33; 95% CI, 0.17-0.65; P =.0008).
These findings were consistent regardless of the ALK tyrosine kinase inhibitor that was used, with a pooled median PFS of 9.2 months (95% CI, 7.4-13.1) in the cohort of patients with concomitant alterations compared with 27.9 months (95% CI, 19.9-43.4) in the group with only ALK alterations (HR, 0.42; 95% CI, 0.24-0.73; P =.0017).
Data from in vitro study indicated that the loss of p53 function due to TP53 mutations in NSCLC cell lines with EML4–ALK fusions resulted in decreased expression of proapoptotic proteins after treatment with alectinib. Introduction of wild-type TP53 into the cell lines restored alectinib efficacy. The addition of ixazomib also restored the efficacy of alectinib in cell lines through the expression of the proapoptotic protein Noxa.
Combination ixazomib plus alectinib therapy in immunodeficient mice with subcutaneous NSCLC tumors and concomitant ALK and TP53 alterations resulted in substantial tumor shrinkage, with 3 of 8 tumors completely regressing. In the group treated with only alectinib, tumor shrinkage was initially modest; the tumors later regrew.
“Concomitant TP53 mutations were highly correlated with unfavorable efficacy of alectinib in patients with TKI-naïve ALK-rearranged NSCLC…and the combined use of a proteasome inhibitor with alectinib restored the unfavorable efficacy in our preclinical models,” the study authors stated. These data should be validated in clinical trials, they concluded.
Disclosures: Some of the study authors disclosed financial relationships with the pharmaceutical industry and/or the medical device industry. For a full list of disclosures, please refer to the original study.
Reference
Tanimoto A, Matsumoto S, Takeuchi S, et al. Proteasome inhibition overcomes ALK-TKI resistance in ALK-rearranged/TP53-mutant NSCLC via Noxa expression. Clin Cancer Res. Published online December 11, 2020. doi:10.1158/1078-0432.CCR-20-2853