Abstract
Background
Short-acting ß2-agonist (SABA) rescue therapy can relieve chronic obstructive pulmonary disease (COPD) symptoms. We assessed post-randomisation treatment effects on exacerbations and health-related quality of life in ETHOS by rescue SABA use.
Methods
In ETHOS (NCT02465567), symptomatic people with COPD and an exacerbation history were randomly assigned 1:1:1:1 to budesonide/glycopyrronium/formoterol fumarate dihydrate (BGF; 320/14.4/10 or 160/14.4/10 μg), glycopyrronium/formoterol fumarate dihydrate (GFF; 14.4/10 μg), or budesonide/formoterol fumarate dihydrate (BFF; 320/10 μg). Post-hoc analyses assessed exacerbation rates by baseline and post-randomisation SABA use (>4 versus ≤4 inhalations/day), St George's Respiratory Questionnaire change from baseline by post-randomisation SABA use (>4 versus ≤4 inhalations/day), and post-randomisation SABA use surrounding (30 days before, day of onset, 30 days after) the first exacerbation.
Results
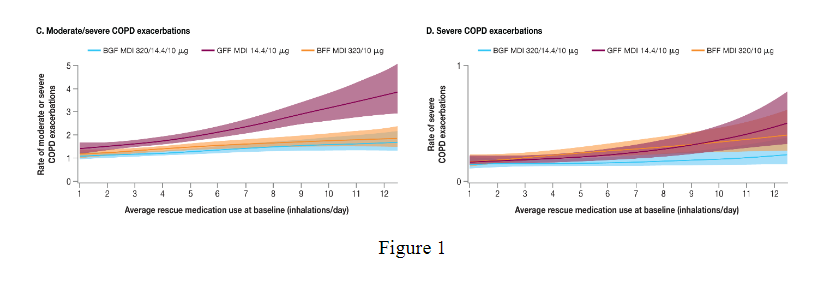
Across treatments, higher moderate/severe exacerbation rates were observed for participants with higher (range: 1.62–2.51) versus lower (range: 1.14–1.51) SABA use at baseline or post-randomisation. Post-randomisation SABA use increased in the 30 days preceding, and decreased in the 30 days following, an exacerbation. Evidence of BGF benefit versus dual therapies in reducing moderate/severe exacerbation rates were seen regardless of SABA use level at baseline or post-randomisation, with greater BGF benefit observed versus GFF with higher SABA use (rate ratios [95% CI]: high baseline SABA, 0.62 [0.53, 0.72]; high post-randomisation SABA, 0.64 [0.54, 0.76]).
Conclusion
These results suggest increased SABA use is associated with an impending exacerbation. Further, BGF reduces exacerbation rates regardless of SABA use, with greater benefit in those with higher SABA use.














