Abstract
Background
This study explores the effectiveness and safety of microbiome-directed-antimicrobial-therapy versus usual-antimicrobial-therapy in adult cystic fibrosis pulmonary exacerbations.
Methods
A multi-centre two-arm parallel randomised control trial conducted across Europe/North-America enrolled 223 participants (January 2015 - August 2017). All participants were chronically colonised with Pseudomonas aeruginosa and were randomised 1:1 into two study-arms. The “usual-therapy group” received 2-weeks of IV ceftazidime 3g thrice-daily (for allergies: aztreonam 2g thrice-daily) and tobramycin 5–10mg·kg−1 once-daily. The “microbiome-directed group” received the same usual-therapy plus an additional antibiotic with greatest presumed activity against the 2nd, 3rd and 4th most abundant genera present in the sputum microbiome, selected by a Consensus Expert Treatment Panel. The primary outcome was change in percentage of predicted FEV1 (ppFEV1) at 14 days post initiation of antibiotics. Secondary outcomes examined ppFEV1 at 7 days, 28 days, and 3 months; time-to-next exacerbation; symptom burden at 7 days; Health Related Quality of Life (HRQoL) at 28 days; and number of exacerbations and IV antibiotic days at 12 months.
Results
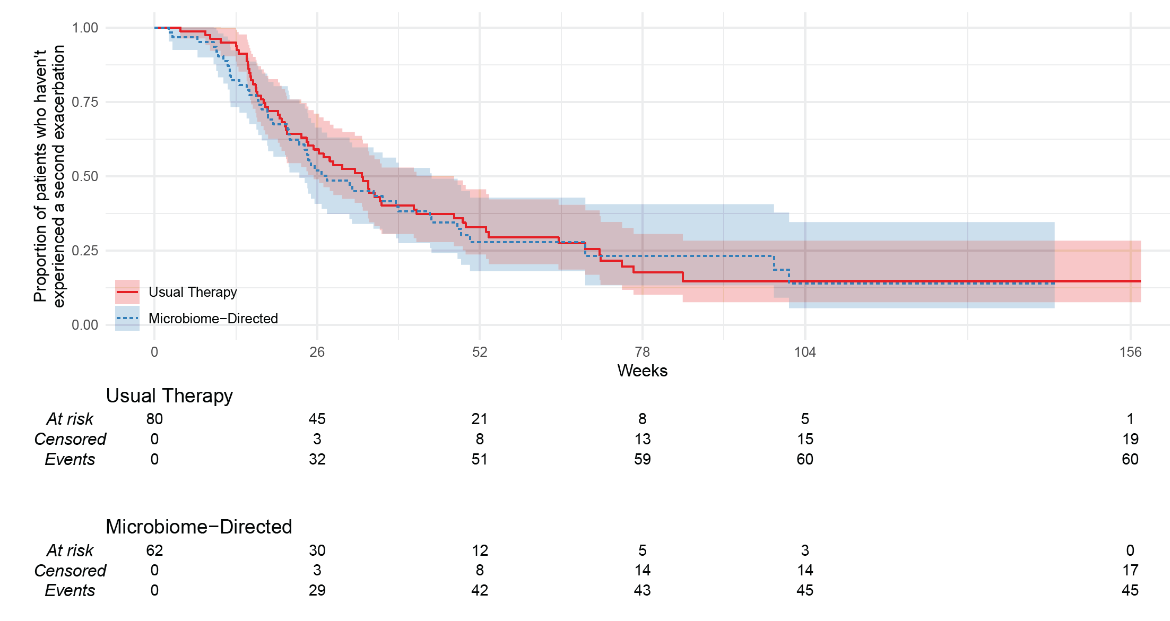
149 participants had an eligible exacerbation (usual-therapy n=83, microbiome-directed therapy n=66). There was no difference between the groups for ppFEV1 at day 14 (−1.1%, 95%CI −3.9 to 1.7; p=0.46), or ppFEV1 measured at other time-points, or for time-to-next exacerbation (microbiome-directed versus usual-therapy Hazard Ratio 0.91 [95%CI 0.60 to 1.38; p=0.66]). The microbiome-directed group trended to have more IV days (median 42 versus 28; p=0.08) and more subsequent exacerbations (median 3 versus 2; p=0.044) the following year. There were no appreciable differences in symptom burden; however, HRQoL sub-scores were consistently worse in the microbiome-directed group (−4.3 points versus usual therapy (95%CI −8.3 to −0.3, p=0.033).
Conclusion
The addition of a third antibiotic based on sputum microbiome sequencing analysis did not result in improved clinical outcomes.