Abstract
Most patients in real-world severe asthma populations would not be eligible for biologic randomised controlled trials (RCTs), although observational evidence has confirmed the effectiveness of biologics in real-world populations. We therefore investigated whether satisfying specific RCT inclusion/exclusion criteria affects biologic response in the real world.
Inclusion and exclusion criteria from 11 pivotal phase 3 asthma biologics RCTs were reviewed to identify criteria themes, and median stringency within each characterised. Patients within the UK Severe Asthma Registry (UKSAR) with at least one year of follow-up on biologics were assessed as to whether they would satisfy inclusion/exclusion for each theme. Regression models were undertaken to assess whether the proportion of patients achieving a composite biologic response, defined as a≥50% reduction in exacerbations or maintenance oral corticosteroids, was non-inferior in patients ineligible by each theme. Superiority analyses and domain specific responses were also analysed.
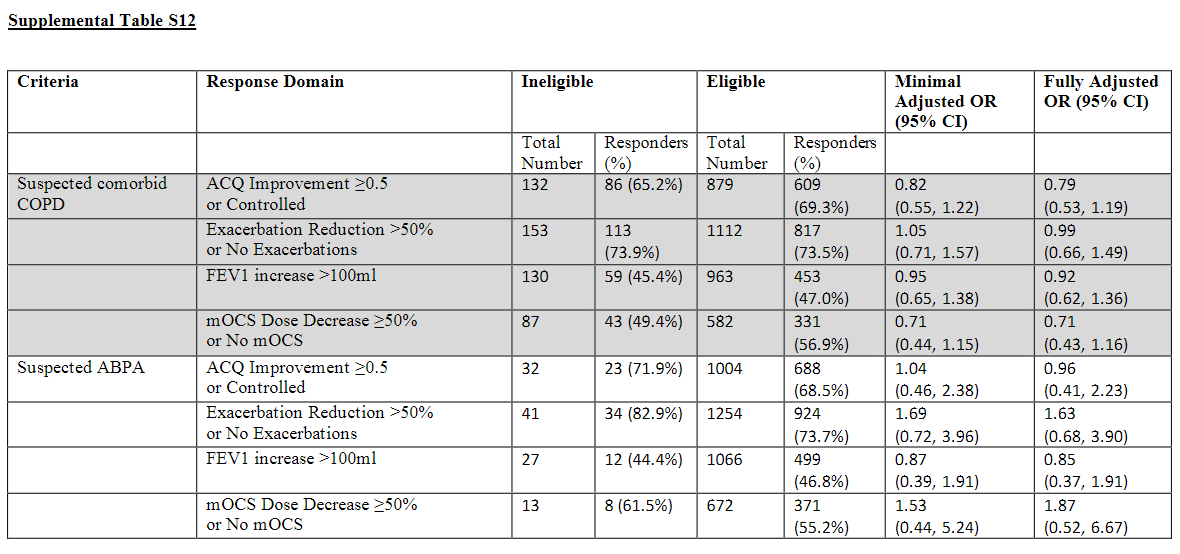
1421 adult patients with severe asthma from 13 specialist centres were included in this analysis. Non-inferiority of composite response was demonstrated for all eligibility criteria except medication adherence. In superiority analyses, patients ineligible by adherence theme had a significantly lower Odds Ratio (OR) for composite response of 0.37 (95% CI 0.20, 0.68) whilst patients ineligible by (low) baseline asthma symptom score had a significantly higher OR for response of 2.09 (1.31, 3.32).
Ineligibility by typical RCT inclusion/exclusion themes was generally not associated with inferior biologic composite response. Asthma biologics are effective in a broad range of patients, many who would not have met clinical trial eligibility criteria.