Abstract
Background
Three biologics targeting interleukin 5 (anti-IL-5) or its receptor-? (anti-IL-5R?) are approved for patients with severe asthma.
Methods
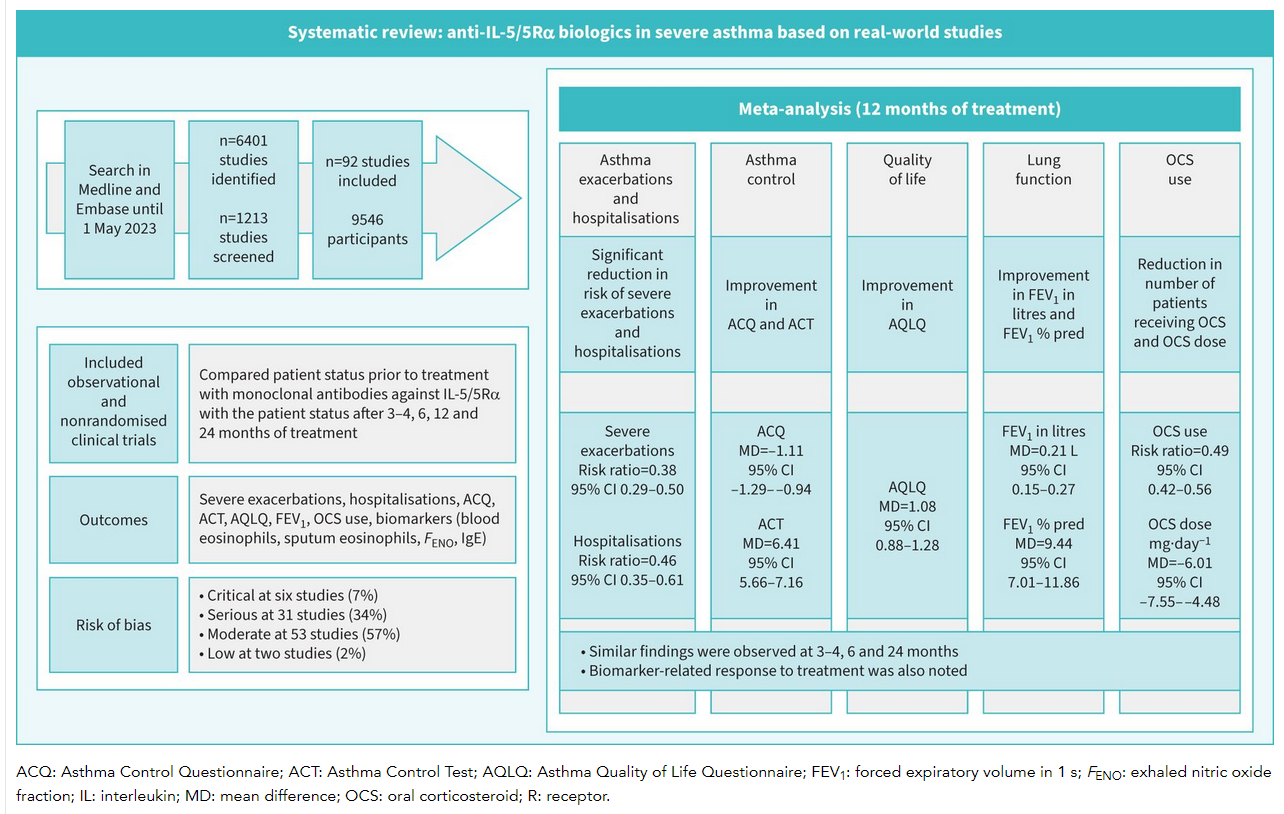
We systematically searched the literature published in Medline and Embase up to 1 May 2023 to identify observational studies and nonrandomised trials that assess the response to anti-IL-5/5R? in real-life patients with severe eosinophilic asthma. We also performed random-effects meta-analyses.
Results
We identified 6401 studies, of which 92 with 9546 patients were analysed. Biologics use was associated with a 62% reduction in severe exacerbations (risk ratio 0.38, 95% CI 0.290.50) and a 54% reduction in hospitalisations (risk ratio 0.46, 95% CI 0.350.61) at 12?months of treatment, compared to pre-treatment. Biologics improved asthma control (decrease in asthma control questionnaire score by 1.11 points (95% CI ?1.29?0.94) and increase in asthma control test score by 6.41 points (95% CI 5.667.16)) and increased the asthma quality of life questionnaire score by 1.08 points (95% CI 0.881.28) and forced expiratory volume in 1?s by 0.21?L (95% CI 0.150.27) at 12?months. There was a significant reduction in oral corticosteroids use of 51% (risk ratio 0.49, 95% CI 0.420.56), with a mean dose reduction of 6.01?mg·day?1 (95% CI ?7.55?4.48) at 12?months of treatment. Similar findings were observed at 34, 6 and 24?months. A biomarker-related response to treatment was also noted.
Conclusions
This comprehensive meta-analysis summarises the significant clinical response to anti-IL-5/5R? biologics in real-life studies, providing important insights for their use in clinical practice.